GEOS Composition Forecasting System (GEOS-CF): Capabilities and complexities
Visualization: Greg Shirah (NASA/GSFC SVS) and Trent L. Schindler (USRA/GESTAR SVS)
Simulating the chemical formation (and destruction) of air pollutants is a highly complex task that involves hundreds of chemical species reacting with each other simultaneously in our atmosphere. A new visualization produced by the NASA Scientific Visualization Studio (SVS) shows the complexity of the chemical system of the atmosphere as simulated by the NASA GEOS composition forecast model (GEOS-CF), produced by the GMAO. In near real-time, GEOS-CF keeps tab of the three-dimensional structure of 250 chemical species—96 of which are shown in the visualization. These compounds occur in tiny concentrations—many times less than 1 part per trillion—yet their abundance is critical to the formation of major health-relevant air pollutants such as ozone.
Low ozone concentrations are shown in dark blue and high concentrations, found in the afternoon near highly populated areas, are shown in white.
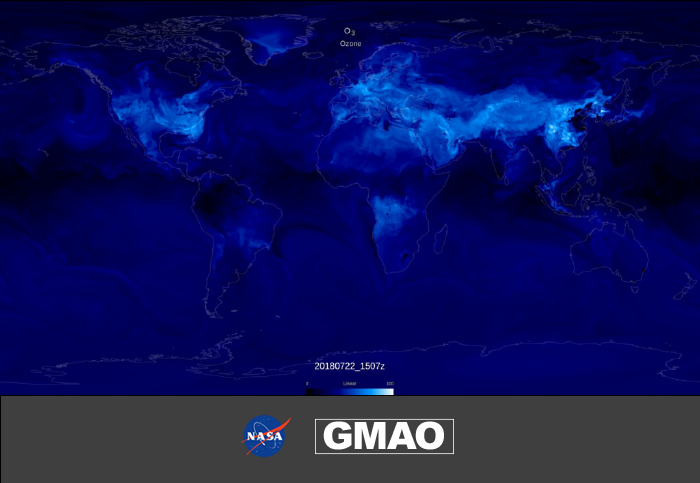
The visualization starts out by showing the surface concentration of ozone, as simulated by GEOS-CF for a few days in mid-summer 2018. Low levels of ozone are shown in dark blue and generally found over the remote oceans. As ozone concentrations increase, the GEOS-CF ozone transitions to white (scale maximum is 100 parts per billion by volume (ppbv) and greater). High values of ozone usually correspond with the peak daylight hours and where there is an abundance of primary pollutants from fossil fuel combustion, biomass burning, or vegetation. The animation marches through a stream of 95 species (which is not even half of the chemical species simulated in GEOS-CF!), which play a role in ozone chemistry, featured as the different "families" or groups of chemical species: the Ox family, extended HOx family, hydrocarbons, “isoprene oxidation”, aerosols, and the extended NOx family. The animation finally ends back at ozone, about two months later.
Highlighting some of the big players in the animation: The hydroxyl radical, OH, a member of the HOx family, is known as the atmosphere's "cleansing agent". It is highly reactive with hydrocarbons like carbon monoxide (CO) and methane (CH4). OH has an extremely short lifetime which makes it difficult to directly measure and simulate. Isoprene is the most abundant "biogenic" VOC, of which there are many very similar compounds emitted by the different trees and shrubs covering the Earth’s surface. The reaction of these chemicals emitted by the plants’ leaves into the atmosphere with sunlight can cause a blue haze in areas with thick vegetation, leading to colloquial names such as the "Smoky Mountains" or "Blue Mountains". In addition, these biogenic compounds can also play a role in the formation of "aerosols", tiny particles suspended in the air that depending on their size and make-up can be damaging to human health if breathed in. There are other natural aerosols, including sea spray, dust and particles injected into the atmosphere from volcanic eruptions or wildfires.
During the time period covered by the animation, there were major fires in California, most notably the Carr Fire and Mendocino Complex Fire (both late July 2018; Camp, Hill and Woolsey Fires were in November 2018). These fires significantly altered the chemical composition of the atmosphere for hundreds of miles around the origin of the fire. Though the majority of aerosols globally have natural sources, the anthropogenic aerosols (including from biomass and biofuel burning, fossil fuel combustion, and application of fertilizer) will impact a greater portion of the population because of close proximity to the sources.
Along with aerosols and ozone, nitrogen dioxide is one of the primary air pollutants for health studies. Nitrogen dioxide is produced naturally by lightning and wildfires; however, its largest concentrations are found close to some of its primary anthropogenic emission sources (it is a by-product of fossil fuel combustion), which is in highly populated areas and along shipping tracks, as simulated by the GEOS-CF.
Simulating ozone chemistry correctly is still an active area of research; there are still many unknown processes in atmospheric chemistry and either unknown or misrepresented pollutant emission sources which lead to model biases in the simulated ozone when compared to ground measurements of ozone. The GEOS-CF system, which is being developed jointly with several government and non-profit partners, offers a new tool to atmospheric chemistry scientists and the health and air quality communities alike.
96 chemical species are shown from a GEOS atmospheric simulation: https://svs.gsfc.nasa.gov/4754
Simulation of Surface Ozone: https://svs.gsfc.nasa.gov/4764

